Adsorption Measurement of Activated Carbon
The IDS system can be used to analyze and measure the adsorption capacity of activated carbons for various volatile organic compounds (VOCs) and other contaminants such as ammonia, sulfur compounds, mercury, amines, and methylene blue. This makes IDS useful as a powerful tool for quantifying the efficacy of various filtration systems and materials in a wide range of industries and applications, including drinking water filtration, flue gas filtration, and chemical filtration systems that are used to protect microelectronic devices and data storage drives.[iv][v] Using this instrument, an adsorption isotherm can be obtained. An adsorption isotherm is a graph that represents the variation in the amount of adsorbate adsorbed on the surface of the adsorbent with the change in concentration at a fixed temperature. This capability can be used to evaluate and compare different filtration systems for their effectiveness at removing environmental hazards such as VOCs and methylene blue (MB), for example. The properties of MB are built into the current IDS database and is considered among various toxic dyes that possess carcinogenic and non-biodegradable properties that can cause a threat to human and animal health. The IDS system can be used and customized to compare different commercial activated carbon materials with respect to their adsorption capacity to such contaminants as well as their tendency to release soluble reactive ions into the environment that can cause interference and/or precipitation problems in water or when used in various filtration systems.
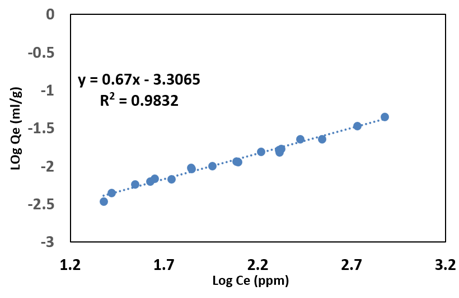
Adsorption amount (Qe) as a function of concentration (Ce)
[iv] Erol Ayranci & Osman Duman,“In-Situ UV-Visible Spectroscopic Study on the Adsorption of some Dyes onto Activated Carbon Cloth”, Journal Separation Science and Technology, Volume 44, 2009 – Issue 15
[v] Fuchs F, Sontheimer H., “Use of ultraviolet (UV) absorption for control of adsorption processes.”, J Environ Pathol Toxicol Oncol. 1987 Sep-Dec;7(7-8):393-402.